inversely proportional to effective nuclear charge
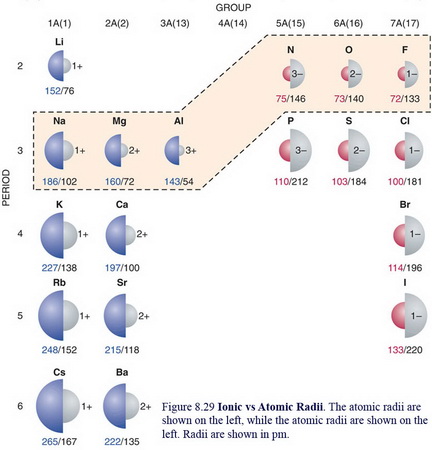
Ionic radius is the distance from the nucleus of an ion up to which it has an influence on its electron cloud. Ions are formed when an atom loses or gains electrons. When an atom loses an electron it forms a cation and when it gains an electron it becomes an anion. .Metallic radii for 12-coordination are given for all metals. Covalent radii are in parentheses. Ionic radii are for six-coordination. Ionic radii ∝ 1 Z e f f Z e f f → effective nuclear charge This Z e f f is calculated as follows: Z e f f = Z - screening constant (σ). Ionic radii are measured by proportioning ionic bond lengths between two ions within a crystal lattice. After studying many compounds, Linus Pauling found that the approximate ionic radii of O 2- was 140pm. With this reference point, Pauling was able to calculate the ionic radii of other ions. Ionic radii are not fixed properties of ions. Atomic and ionic radii are found by measuring the distances between atoms and ions in chemical compounds. On the periodic table, atomic radius generally decreases as you move from left to right across a period (due to increasing nuclear charge) and increases as you move down a group (due to the increasing number of electron shells). Similar trends are observed for ionic radius, although.
Binversely proportional to square of effective nuclear charge
Cdirectly proportional to effective nuclear charge
Ddirectly proportional to square of effective nuclear charge
Solution:
Ionic Radii Are Found
Ionic radii $∝ frac{1}{Z_eff}$
$ , , , , , , , , , Z_{eff} rightarrow , , , $> effective nuclear charge
This $Z_{eff}$ is calculated as follows :
$Z_{eff}=Z$- screening constant $(sigma)$
This value of screening constant is based upon the number of electrons in valence shell as well as in penultimate shells.
1. The correct statement in respect of protein haemoglobin is that it
2. The hormone that helps in the conversion of glucose to glycogen is
3. The frequency of radiation emitted when the electron falls from n = 4 to n=1 in a hydrogen atom will be (Given ionisation energy of $H = 2.18times 10^{18} $J $atom^{-1}$ and$h = 6.625 times 10^{-34} Js)$
4. The enzyme which hydrolysis triglycerides into fatty acids and glycerol is called
5. Which of the following will not form a yellow precipitate on heating with an alkaline solution of iodine?
6. Ionic radii are
7. Among $[Ni(CO)_4], [Ni(CN)_4]^{2-} , [NiCl_4]^{2-}$ species, the hybridisation states of the Ni atom are, respectively (At. no. of Ni = 28)
Ionic Radii Periodic Table
8. Considering $H_2O$ as a weak field ligand, the number of unpaired electrons in $[Mn(H_2O )_6]^{2+} $ will be (At. no. of Mn = 25)
9. Which of the following does not have a metal-carbon bond?
10. The rate of first order reaction is $1.5 times 10^{-2}$ mol $L^{-1} , min^{-1}$ at 0.5 M concentration of the reactant. The half-life of the reaction is
Questions from Classification of Elements and Periodicity in Properties
1. The group number, number of valence electrons, and valency of an element with atomic number 15, respectively, are
2. The first electron affinity of C, N and O will be of the order
3. The electronic configuration of the atom having maximum difference in first and second ionization energies is
4. The 3rd period or the periodic table contains
Ionic Radii Chart
5. The element californium belongs to the family of
6. Which of the following belong to the category of transition metal ?
7. Elements with atomic number 35 belongs to
8. To which block of the periodic table does element with atomic number 56 belong
9. The tenth element in the periodic table resembles the element with atomic number
10. Compared to the first ionisation potential, the value of second ionisation potential of an element is
1. In Wolff‐Kishner reduction, the carbonyl group of aldehydes and ketones is converted into
2. Identify compound X in the following sequence of reactions:
3. Identify a molecule which does not exist.
4. Identify the incorrect match.
Name IUPAC Official Name A Unnilunium i Mendelevium B Unniltrium ii Lawrencium C Unnilhexium iii Seaborgium D Unununnium iv Darmstadtium
| Name | IUPAC Official Name | ||
|---|---|---|---|
| A | Unnilunium | i | Mendelevium |
| B | Unniltrium | ii | Lawrencium |
| C | Unnilhexium | iii | Seaborgium |
| D | Unununnium | iv | Darmstadtium |
5. Reaction between acetone and methyl magnesium chloride followed by hydrolysis will give :
6. Identify the correct statements from the following:
(a) $CO_2(g)$ is used as refrigerant for ice-cream and frozen food.
(b) The structure of $C_{60}$ contains twelve six carbon rings and twenty five carbon rings.
(c) $ZSM-5$, a type of zeolite, is used to convert alcohols into gasoline.
(d) $CO$ is colorless and odourless gas.
7. Which of the following set of molecules will have zero dipole moment ?
8. On electrolysis of dil.sulphuric acid using Platinum (Pt) electrode, the product obtained at anode will be:
9. An element has a body centered cubic (bcc) structure with a cell edge of 288 pm. The atomic radiusis:
10. Find out the solubility of $Ni(OH)_2$ in 0.1 M NaOH. Given that the ionic product of $Ni(OH)_2$ is $2 times 10^{-15}$
